Health & Education
Tribe begins administering doses of Moderna COVID-19 vaccine
By Danielle Harrison
Smoke Signals staff writer
The Confederated Tribes of Grand Ronde started administering the highly anticipated COVID-19 vaccine just before Christmas.
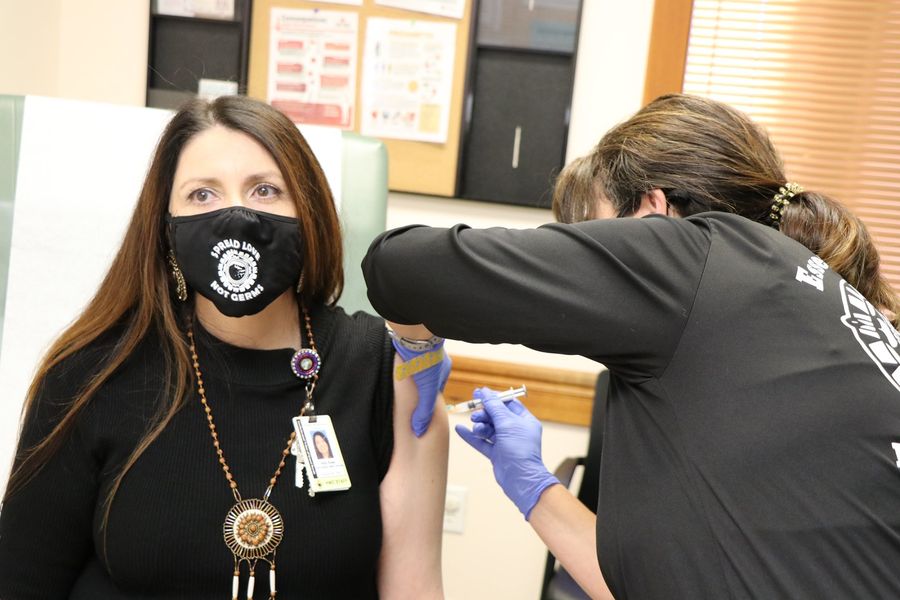
Health Services Executive Director Kelly Rowe said the Tribe's Health & Wellness Center received 200 doses of the recently approved Moderna vaccine on Tuesday, Dec. 22.
“I am very happy to say we received the first allotment of the Moderna vaccine and we are starting to vaccinate clinic personnel, Tribal first responders, then adult foster care workers and residents, so we can start getting some herd immunity built up,” she said.
As of 2 p.m. Wednesday, Dec. 23, approximately 20 essential health care workers at the Tribe had received their first dose of the vaccine, including Rowe.
“I had mine this morning and had no side effects,” Rowe said.
She said that only 20 doses would be administered per day in case side effects resulted in essential clinic personnel having to stay home sick.
“We don’t want to potentially have an entire department out,” she said. “We have identified staff who want the vaccine as priority and working toward the ones who are ‘maybes’ and then reaching out to first responders.”
The Tribe is coordinating with the Oregon Health Authority for vaccination delivery. Those who receive first doses need to have the follow-up dose 28 days later.
Rowe advised anyone with questions about vaccination to visit the Tribe’s COVID-19 web page at www.grandronde.org or the Tribe’s Facebook Page.
“Please do not call the clinic because it ties up the line for people who are trying to get in for COVID testing,” she said. “We will announce updates as prolifically as possible. We have gotten the vaccine, and we will continue to get it.”
The Confederated Tribes of the Umatilla's Yellowhawk Health Center was among the first medical facilities in Oregon to receive the Pfizer-BioNTech COVID-19 vaccine.
The Umatilla Tribe has had approximately 8 percent of its COVID-19 tests come back positive since the pandemic hit. The state average is approximately 5.8 percent.
The Umatilla Reservation also is surrounded by Oregon’s Umatilla and Union counties, which have had some of the worst virus outbreaks in the state, according to a recent Oregon Public Broadcasting article.
In order to store the vaccine, which requires ultra-low temperatures, the Umatilla Tribe procured a freezer that was previously used to keep lamprey specimens for study. The Moderna vaccine can be stored at regular refrigeration temperatures.
Grand Ronde’s Health & Wellness Center coronavirus cases are below the state average, with approximately 3.2 percent of tests coming back positive.
The first COVID-19 vaccine doses arrived in Oregon on Monday, Dec. 14, and Legacy Health was the first registered vaccine provider in the state to receive them. Sites in Portland and Tualatin each took delivery of one package of 975 doses, according to a press release from the Oregon Health Authority.
Additional doses were expected at three other locations in Oregon on Tuesday, Dec. 15: Oregon Health & Science University Pharmacy, a Kaiser Permanente site in Portland and St. Alphonsus Medical Center in Ontario.
The remaining 30,225 vaccine doses from Oregon’s first-week allocation arrived at hospitals throughout the rest of the week, with 10,725 of the doses going to nursing facilities. Approximately 10,407 people were vaccinated during the first week, according to the Health Authority.
The federal Centers for Disease Control and Prevention asked Oregon to choose the sites as a way to test the ordering system.
The long-anticipated vaccine shipments followed a U.S. Food and Drug Administration decision on Friday, Dec. 11, to issue an emergency use authorization for the Pfizer-BioNTech vaccine. It was found in clinical trials to have a 95 percent effective rate, and only cause mild to moderate short-term side effects.
"In recent weeks, as COVID-19 vaccines reached the final stages of approval, I have said time and again that hope is on the way,” Gov. Kate Brown said. “Today, I can tell you that help is here.”
Vaccinations will be prioritized, starting with frontline health care workers and long-term care facility residents who are among the most vulnerable.
"Throughout the process, we will work to ensure that the Oregonians that have been disproportionately impacted by COVID-19, including those from Black, Indigenous, Latino/Latina/Latinx, Pacific Islander and Tribal communities, have equitable access to vaccination,” Brown said.
Oregon Health Authority Director Patrick Allen said that vaccinations against COVID-19 are still months away for most Oregonians, so prevention measures such as wearing masks, physical distancing, avoiding gatherings and staying home if sick must continue.
The vaccine manufactured by Moderna Inc. received FDA emergency use authorization on Friday, Dec. 18. The Moderna vaccine has been found to be approximately 94 percent effective with minimal side effects.
Public health officials estimate there will be enough of the two vaccines to provide first doses to about 100,000 people, with second doses administered in January. People will need to follow public health protocols between the two doses and up to several weeks following the second dose as their bodies build up resistance to the virus.
Essential workers, followed by people with underlying health conditions and those older than 65 are next in line as they are identified by the Oregon Health Authority’s Vaccine Advisory Committee.
Priority groups in Phase 2 will be determined at a later date, and the general population isn’t expected to be eligible for vaccination until spring.
